Achievements
TRANSVAC, a success story
The key to successful vaccination lies within a specialised chain of events, beginning with the identification of a public health need, and ending in an effective and affordable vaccine accessible by those most in need of it. In between these milestones lies a complex set of highly skilled practical and administrative steps, governed by constantly-evolving regulatory frameworks, all of which are ultimately defined by the need for significant financial and technical resources. It is within this ecosystem of events that vaccine scientists must address a huge number of technical, regulatory and financial challenges.
Supporting and uniting these scientists for the benefit of Europe and its citizens is the focus of the EU-funded project TRANSVAC. In its first funding period, which commenced in 2009 and was completed in 2013, TRANSVAC made a significant contribution to the support of individual European scientific groups through the provision of state-of-the-art vaccine experimental services, technical training, and innovative vaccine research and development (R&D). Some 50 vaccine candidates in 29 vaccine projects were accelerated by TRANSVAC, and many continue to this day along the pathway towards licensure.
Furthermore, over thirty scientists received specialised training on numerous aspects of vaccine development, being provided with a thorough overview of the full vaccine development process, and more than twenty innovative scientific papers were published from TRANSVAC’s research and activities (for more information see also tables below and: Geels, M. et al (2015): TRANSVAC research infrastructure – Results and lessons learned from the European network of vaccine research and development. Vaccine 33, 5481-5487. doi: 10.1016/j.vaccine.2015.01.079).
Another significant achievement of TRANSVAC in its first funding period was to develop a roadmap to define a coherent and strategy-led approach to address the existing fragmentation of European vaccine development, based on the unification of leading European vaccine experts, so as to establish a “European vaccine R&D infrastructure”. In order to address the fragmentation of vaccine R&D in Europe, transnational cooperation between existing organisations from the public and private sectors was deemed to be essential, and the establishment of a European vaccine R&D infrastructure agreed to be a critically important step. Additional confirmation for the need to establish a European vaccine infrastructure came from the infrastructure needs analysis that was performed as part of the “Innovation Partnership for a Roadmap on Vaccines in Europe” (IPROVE), resulting in a European vaccine roadmap (launched in March 2016).
This European vaccine R&D infrastructure need is met by the continuation of TRANSVAC, with the aim of establishing a sustainable European vaccine R&D infrastructure.
TRANSVAC, major achievements:
The major achievements of the funding period 2009 – 2013 of TRANSVAC include:
-
Vaccine adjuvant formulation studies for 50 new vaccine antigen/adjuvant combinations.
-
Preparation, curatorship and shipping of 164 specified reference standards (vials) for use in vaccine development.
-
Evaluation of four vaccine candidates in animal models, including non-human primates (NHP), pigs and mice, to gain insight into the active mechanisms of each vaccine candidate.
-
Research on vaccine biomarkers, including in-depth comparative analysis of data from different platforms, large-scale RNA sequencing, harmonisation of standard operating procedures (SOPs) for sample, microarray and data analysis, as well as transcriptome mapping (more than 1400 samples analysed).
-
Harmonisation and standardisation of vaccine assays, providing reproducible data for the selection of formulation strategies, administration routes, dosages, and schedules.
-
Standardised and harmonised SOPs for immunoassays used in clinical trials were made available.
-
Preparation and distribution of 19 reference antigens for several diseases including malaria, HIV and tuberculosis, and the establishment of five new cell banks to improve the growth of adenoviral vectors.
-
Compilation of an investigational medicinal product dossier (IMPD) for the clinical trial application for two candidate vaccines, allowing them to proceed to phase I clinical trials.
-
Organisation of two modular courses in vaccine development, and six workshops in diverse vaccine-related subjects such as statistical analysis, animal models and global analysis.
-
All courses were rated as “excellent” or “very good” by every single one of the more than thirty trainees.
-
Preparation of a European vaccine roadmap outlining the strategy towards the establishment of a sustainable European vaccine R&D infrastructure.
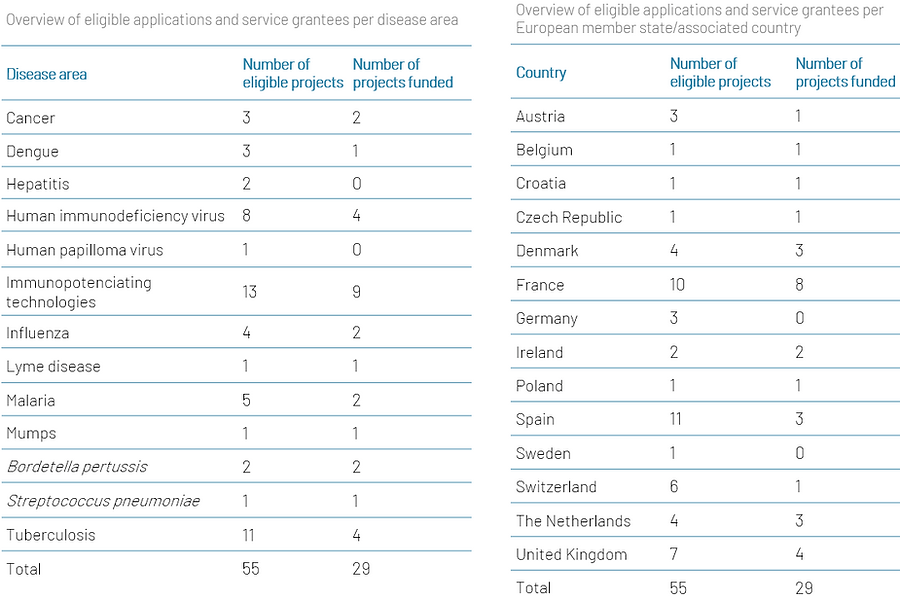
TRANSVAC in numbers...
Approximately half (53%) of all eligible projects were selected to receive support, with Spain and France submitting the most applications.
TRANSVAC in publications...
2017
-
Smith SG, Harris SA, Satti I, Bryan D, Walker KB, Dockrell HM, McShane H and Ho MM. 2017. Assay optimisation and technology transfer for multi-site immuno-monitoring in vaccine trials. PloS One 12 (10): e0184391.
2015
-
Geels M, Thøgersen RL, Guzman CA, Ho MM, Verreck F, Collin N, Robertson JS, McConkey SJ, Kaufmann SH, Leroy O. TRANSVAC research infrastructure - Results and lessons learned from the European network of vaccine research and development. Vaccine. 2015 Oct 5;33(41):5481-7. doi: 10.1016/j.vaccine.2015.01.079. Epub 2015 Feb 8
-
Michalis Kotsyfakis, Petr Kopáček, Zdeněk Franta, Joao H. F. Pedra, José M. C. Ribeiro. Deep Sequencing Analysis of the Ixodes ricinus Haemocytome. PLOS Neglected Tropical Diseases DOI:10.1371/journal.pntd.00037
-
Kotsyfakis M, Schwarz A, Erhart J, Ribeiro JM. Tissue- and time-dependent transcription in Ixodes ricinus salivary glands and midguts when blood feeding on the vertebrate host. Sci Rep. 2015 Mar 13;5:9103. doi: 10.1038/srep09103
2014
-
Schwarz A, Tenzer S, Hackenberg M, Erhart J, Gerhold-Ay A, Mazur J, Kuharev J, Ribeiro JM, Kotsyfakis M. A systems level analysis reveals transcriptomic and proteomic complexity in Ixodes ricinus midgut and salivary glands during early attachment and feeding. Mol Cell Proteomics. 2014 Oct;13(10):2725-35. doi: 10.1074/mcp.M114.039289. Epub 2014 Jul 21
-
Sanchez MV, Ebensen T, Schulze K, Cargnelutti D, Blazejewska P, Scodeller EA, Guzmán CA. Intranasal delivery of influenza rNP adjuvanted with c-di-AMP induces strong humoral and cellular immune responses and provides protection against virus challenge. PLoS One. 2014 Aug 20;9(8):e104824. doi: 10.1371/journal.pone.0104824. eCollection 2014 (WP3)
-
C. Dutruel, J. Thole, M. Geels, H. Mollenkopf, T, Ottenhoff, C.A. Guzman, H.A. Fletcher, O. Leroy, S.H.E. Kaufmann. TRANSVAC workshop on standardisation and harmonisation of analysis platforms for HIV, TB and malaria vaccines. ‘How can big data help?’. Vaccine, 32, issue 35, 31 July 2014, 4365 - 4368 (WP3)
-
O. Leroy, M. Geels, J. Korejwo, B. Dodet, N. Imbault, S. Jungbluth. Roadmap for the establishment of a European vaccine R&D infrastructure. Vaccine 32, issue 51, 5 December 2014, pages 7021 - 7024
2013
-
Misstear K. McNeela EA, Murphy AG, Geoghegan JA, O'Keeffe KM, Fox J, Chan K, Heuking S, Collin N, Foster TJ, McLoughlin RM and Lavelle EC. (2013). Targeted nasal vaccination provides antibody independent protection against Staphylococcus aureus. The Journal of Infectious Diseases, Nov 22, 2013 doi:10.1093/infdis/jit636
-
Weiner J. Maertzdorf J, Kaufmann SH. (2013). The dual role of biomarkers for understanding basic principles and devising novel intervention strategies in tuberculosis. Annals of the New York Academy of Sciences, 1283: 22-29. doi:10.1111/j.1749-6632.2012.06802.x (WP3)
-
Kaufmann S. H. E. and Dorhoi A. (2013). Inflammation in tuberculosis: interactions, imbalances and interventions. Current Opinion in Immunology, 25(4): 441-449. doi:10.1016/j.coi.2013.05.005 (WP3)
-
Kaufmann S. H. E. Tuberculosis vaccines: Time to think about the next generation. Seminars in Immunology, 25(2): 172-181. doi:10.1016/j.smim.2013.04.006 (WP3)
-
P. Riese, K. Schulze, T. Ebesen, B. Prochnow, C. A. Guzman. Vaccine adjuvants: key tools for innovative vaccine design. Curr Top Med Chem, 2013; 13 (20): 2562 - 80 (WP3)
2012
-
Maertzdorf J, Weiner J 3rd, Kaufmann SH. Enabling biomarkers for tuberculosis control. Int J Tuberc Lung Dis. 2012 Sep;16(9):1140-8. doi: 10.5588/ijtld.12.0246 (WP3)
-
Rueckert C, Guzmán CA. Vaccines: from empirical development to rational design. PLoS Pathog. 2012;8(11):e1003001. doi: 10.1371/journal.ppat.1003001. Epub 2012 Nov 8 (WP3)
-
Libanova R. Becker PD, Guzmán CA. Cyclic di-nucleotides: new era for small molecules as adjuvants. Microb Biotechnol. 2012 Mar;5(2):168-76. doi: 10.1111/j.1751-7915.2011.00306.x. Epub 2011 Sep 29 (WP3)
-
Kaufmann SH. Tuberculosis vaccine development: strength lies in tenacity. Trends Immunol. 2012 Jul;33(7):373-9. doi: 10.1016/j.it.2012.03.004. Epub 2012 May 3
-
R.E. Thom, M.J. Elmore, A. Williams, S.C. Andrews, F. Drobniewski, P.D. Marsh, and J.A. Tree. The expression of ferritin, lactoferrin, transferrin receptor and solute carrier family 11A1 in the host response to BCG-vaccination and Mycobacterium tuberculosis challenge. Vaccine, vol. 30, issue 21, 2 May 2012, 3159 - 3168 (WP2)
-
J.A. Tree, S. Smith, N. Baker, S. Clark, F.E. Aldwell, M. Chambers, A. Williams, and P.D. Marsh. Method for assessing IFN-c responses in guinea pigs during TB vaccine trials. Letters in Applied Microbiology, vol. 55, issue 4, 295 - 300 Oct. 2012 (WP2)
-
Grode L. Ganoza CA, Brohm C, Weiner J 3rd, Eisele B, Kaufmann SH. (2013). Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine 2012, 31(9): 1340-1348 (WP3)
-
2011
-
Ebensen T, Libanova R, Schulze K, Yevsa T, Morr M, Guzmán CA. Bis-(3',5')-cyclic dimeric adenosine monophosphate: strong Th1/Th2/Th17 promoting mucosal adjuvant. Vaccine. 2011 Jul 18;29(32):5210-20. doi: 10.1016/j.vaccine.2011.05.026. Epub 2011 May 25 (WP3)
-
N. Collin and P. M. Dubois. The Vaccine Formulation Laboratory: A platform for access to adjuvants. Vaccine 2011 Jul, Vol. 29 supplement, A37-39 (WP1)
-
N. D. Huntington, N. L. Alves, N. Legrand, A. Lim, H. Strick-Marchand, JJ. Mention, A. Plet, K. Weijer, Y. Jacques, P. D. Becker, C. Guzman, P. Soussan, D. Kremsdorf, H. Spits, and J. P. Di Santo. IL-15 transpresentation promotes both human T-cell reconstitution and T-cell–dependent antibody responses in vivo. PNAS, 2011, 108 (15) 6217 - 6222 (WP3)



